June 13, 2024
The Latest Innovations and Advancements in Neuroscience
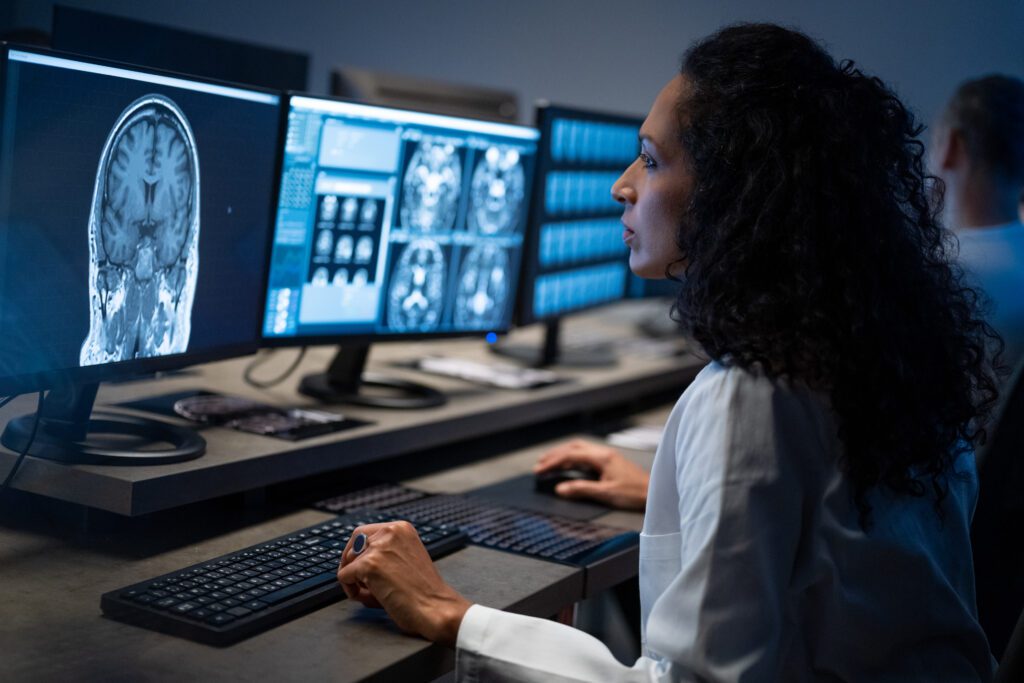
A recent study in The Lancet Neurology shows that neurological conditions are now the leading cause of illness and disability worldwide, and one in three people have a neurological condition, according to the World Health Organization. Alzheimer’s is one of the most debilitating neurological conditions and is difficult to treat and detect early. In the U.S. alone, more than 7 million people are living with the disease and estimates project this number will rise to nearly 13 million people by 2050.Researchers have been working to find new therapies for Alzheimer’s and other neurological conditions, and over the past year progress has been made. Last year, the FDA granted accelerated approval for Leqembi (lecanemab-irmb)—a monoclonal antibody medication developed by Biogen and Eisai Co. that is administered intravenously—to treat Alzheimer’s. Earlier this week, the agency’s Peripheral and Central Nervous System Drugs Advisory Committee recommended Eli Lilly’s new therapy to treat Alzheimer’s, donanemab, for approval. In recognition of Alzheimer’s and Brain Awareness Month this June, we’re highlighting members making breakthroughs in finding treatments for these conditions and advancing other therapies in the neuroscience field.
FDA Approval for a Treatment for a Genetic Neurological Disorder
Neurocrine Biosciences has specialized in finding treatments for rare diseases that affect the brain and endocrine system since it was founded in the early 90s by award-winning scientists from Stanford University and The Salk Institute for Biological Studies. The San Diego-based company recently made headlines when Ingrezza Sprinkle, a new oral granules formulation of its therapy which treats adults with tardive dyskinesia and chorea associated with Huntington’s disease, received FDA approval. Huntington’s disease is a genetic neurological disorder that can cause involuntary movements and motor issues, and the company says a survey noted that patients reported difficulty swallowing pills because of these symptoms. The reformulation allows the contents of the Ingrezza capsule to be sprinkled on soft food, making it easier for patients to take their medication. The company is also conducting a clinical study for a potential new treatment for neurological and neuropsychiatric conditions.
Developing a Blood Test for Alzheimer’s
In recent years, a handful of liquid biopsies that can detect circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA) in a blood sample to help monitor some types of cancers have been FDA approved. What if Alzheimer’s could also be identified in its early stages through a blood draw? That’s a future Superfluid Dx is hoping for. The Bay Area-based company is developing is a blood test for Alzheimer’s and other dementias with a platform that analyzes cell-free mRNA . Members of the company’s executive leadership have been or are affiliated with the Chan Zuckerberg Biohub Network, and the company closed its Series A round of financing at the end of 2023.
Personalized Medicine for Parkinson’s Disease
Aspen Neuroscience is devoted to finding a treatment for Parkinson’s disease using personalized cell replacement therapy that aims to replace damaged neurons in the brain with healthy ones derived from a patient’s own cells. The company recently announced that it dosed the first patient in the ASPIRO trial, a Phase 1/2a clinical trial for ANPD001, an autologous, dopaminergic neuron cell replacement therapy for participants with moderate to severe Parkinson’s disease. “The initiation of this clinical trial is a major milestone in Aspen’s mission to develop and deliver personalized, regenerative neurologic therapies for people with unmet medical needs, starting with Parkinson’s disease,” Damien McDevitt, Ph.D., Aspen Neuroscience president and CEO, said in a release. “To date, there is no disease-modifying therapy that can stop, replace or prevent the loss of dopamine neurons or slow the progression of Parkinson’s.”
Aspen Neuroscience also received a CLIN2 Grant from the California Institute for Regenerative Medicine (CIRM) for $8 million to advance the development of ANPD001.
On the Rise
Cajal Neuroscience was launched in 2022, and the Seattle-based company was named one of Biospace’s top life sciences startups to watch this year for its approach in identifying genetic markers in the progression of neurodegenerative diseases and its industrialized lightsheet microscopy platform , which the company says uses 3D imaging to visualize the effects of a target or therapeutic in the brain. The company is currently focused on Parkinson’s and Alzheimer’s diseases, and just last month it announced a partnership to develop oligonucleotide-based medicines for neurodegenerative diseases.
Big Partnerships and Collaboration
In neuroscience, Bristol Myers Squibb focuses on researching Alzheimer’s, multiple sclerosis and schizophrenia, and the company has been strengthening its pipeline with its recent $14 billion dollar acquisition of Karuna Therapeutics, which has been specializing in developing a new drug to treat schizophrenia in adults, and a collaboration with Prothena. This May, the company announced it will pay Prothena $80 million for the exclusive global licensing of PPRX019, a potential treatment for neurodegenerative diseases with an undisclosed target. The FDA had cleared the investigational new drug (IND) application for PRX019 last December, and Prothena plans to launch a clinical trial later this year.
New Neuroscience Startup
Montara Therapeutics is a startup focused on developing treatments for CNS diseases based on precision medicine and genetically validated targets. The Bay Area-based company is led by Nicholas T. Hertz, Ph.D., who was the founder and chief scientific officer at Mitokinin, which AbbVie acquired last year. Hertz and his team have extensive experience in researching treatments for Parkinson’s disease and pioneering work in Alzheimer’s.