
August 15, 2024
Accelerating Targeted Protein Degradation Research with AI and Machine Learning
Authored by Neil Bence, Ph.D., vice president, head of oncology discovery, Bristol Myers Squibb; and Kai Wang, Ph.D., executive director, informatics and predictive sciences, Bristol Myers Squibb. This article originally appeared on bms.com; republished with permission.
Artificial intelligence (AI) has had a profound impact on the way we live and work. From writing an email to tailoring video recommendations and search engine results, AI is becoming increasingly integrated into how we think about problems and perform daily tasks. But how will this technology transform the future of our health and wellbeing? The answer can be found in the ability to solve increasingly complex problems with greater simplicity and ease, including within the healthcare sector. At pharmaceutical companies, AI and machine learning are becoming an increasingly important component of research and development (R&D), resulting in a deeper understanding of diseases, novel drug targets and the ability to deliver better medicines with faster timelines.
As scientists, we find ourselves at a rare moment in time. The convergence of the rapid evolution underway in targeted protein degradation and advancements in AI mark an inflection point. AI and machine learning are building on decades of innovation in the field of protein degradation and are beginning to accelerate the expansion of the platform’s potential to develop new medicines, previously believed to be impossible, for patients, at an incredible pace.
Researchers are now able to use advanced computational models to examine the vast amounts of data generated in the post-human genome era to help discover new disease targets and accelerate discovery of novel degrader molecules. When these molecules move to the clinic, data and predictive technologies inform patient selection, improve trial design and increase operational efficiencies to get new potential treatment options to patients faster. What’s at stake has captured the attention of the research community, with initial transformational outcomes of protein degraders in multiple myeloma offering a glimpse into the possibilities ahead.
The case for targeted protein degradation
Targeted protein degradation is an exciting approach that allows scientists to remove disease-causing proteins. Specifically designed molecules harness the cell’s naturally occurring protein degradation machinery to remove these proteins. This is a novel approach that enables scientists to target disease-related proteins that were once thought to be “undruggable.”
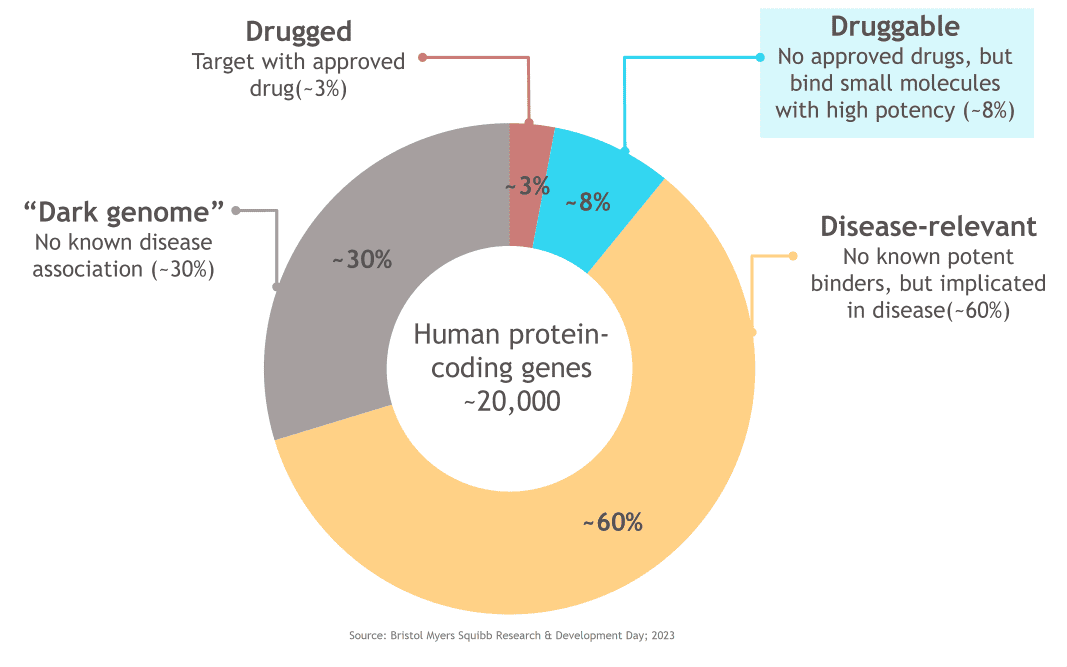
Consider this: current approved medicines target only about 3% of the proteins encoded by the approximately 20,000 human protein-coding genes, reflecting the small fraction of targets that have the potential to bind traditional small molecule therapeutics with high potency. Accounting for genes that have no known disease association, approximately two thirds of disease-relevant proteins lack any potent binders and have yet to be successfully targeted with existing approaches. The potential of protein degradation has inspired scientists by providing access to this vast catalogue of previously believed “undruggable” or difficult to drug proteins.
Bristol Myers Squibb has extensive expertise in the field as the only company to successfully develop and commercialize protein degrader agents. The first degraders were approved based on observations of efficacy in the clinic without a precise understanding of the molecular mechanisms behind the transformative activity observed in patients. Over the last decade, researchers have developed a deep mechanistic understanding of protein degraders, allowing scientists to now intentionally design novel degraders to target a broad range of diseases. Bristol Myers Squibb now has multiple types of investigational protein degraders, including molecular glues, heterobifunctional agents (ligand-directed degraders, LDDs) and degrader antibody conjugates (DACs). Pursuing a range of modalities allows us to intentionally apply the right degrader to “match the modality to mechanism” to get the best possible results for patients.
Targeted protein degradation was already having its own renaissance, with the advancement of numerous degraders to clinical development over the last decade. Now, this approach is rapidly being taken to the next level with advances in computational science redefining expectations and accelerating timelines.
End-to-end data and technology integration
AI and machine learning can recognize patterns and reveal proteins implicated in disease in ways not possible previously. With robust data sets and advanced computational methods at their fingertips, scientists at Bristol Myers Squibb have incredible predictive power to inform the path to clinical proof of concept. For example, AI and machine learning help scientists to:
- Find novel disease targets through a deep understanding of causal human biology: Vast sets of patient data are being explored to develop a deep understanding of diseases. For example, computer vision algorithms based on deep learning neural networks can be used to extract clinically relevant features from abundantly available tissue images and model the molecular characteristics of patients. Additionally, pretrained foundation AI models, akin to the large language models behind ChatGPT, are being built using single-cell and spatial transcriptomics data to model the gene regulatory networks in cells and to predict the effects of specific gene perturbations. This information can then be applied to large real-world patient data sets to help discover new disease targets for protein degraders well beyond the current clinical validation already seen in cancer.
- Invent new degrader medicines faster, matching modality to a molecular mechanism of action: Once we’ve determined that a degrader is the best way to approach a target, scientists now have many AI-enabled tools to assist them in designing and optimizing novel degrader molecules faster. Design of protein degraders requires assembly of 3D structures of protein complexes and small molecule ligands, which could previously only be achieved by labor- and time-intensive protein crystallography or other experimental techniques. Geometric deep learning models, built on existing protein structure databases, are now able to accurately predict protein complex structures and protein-protein and protein-ligand interaction interfaces, potentially enhancing the structure-based design of degraders. Additionally, diffusion-based generative AI approaches can learn from large compound libraries and are being used to design new degrader molecules with drug-like properties and desired function. Finally, closed-loop active learning workflows that integrate AI and machine learning models of generative molecule design and multi-parameter optimization are accelerating design-make-test-learn cycles for drug candidate nomination.
- Accelerate our ability to deliver new medicines with a clear path to clinical proof of concept: Optimization of trial design and biomarker-driven studies are enhancing the clinical development of protein degrader agents. AI has already brought forward many advances in automation of clinical trials, spanning protocol design, regulatory reporting, execution, and data analytics. And we continue to build on these capabilities, including developing industry-leading virtual simulation platforms that can predict and optimize the likelihood of trial success. We are also exploring new ways to collect data and new data sources enabled by AI to help speed clinical trials of protein degrader agents. Through AI, we can leverage real-world data to conduct virtual clinical trials. AI and machine learning are also being used in concert with automated, real-time data collection via AI-based wearables and sensors to accelerate our clinical programs and reduce patient burden in trial participation. With proper consents and authorizations, we collect complex data from several different sources, including imaging data, data collected from wearables, and traditional biospecimen data. This enables the creation of new data library capabilities to inform next-generation molecule design.
What’s ahead?
The integration of AI and machine learning in drug discovery and clinical development is a relatively recent advancement, but these technologies are already making waves in the industry and are poised to accelerate research efforts and transform our ability to invent novel medicines with greater speed and probability of clinical success. Looking forward, we are confident that targeted protein degradation, with a boost from AI and machine learning, will bring truly novel and transformative therapeutic options to patients with serious disease.