
March 23, 2023
Member Spotlight: Caelum Diagnostic Solutions

Caelum Diagnostic Solutions is Taking a Novel Imaging Technology to the Next Level to Make Real-Time Cancer Diagnosis Possible
In celebration of Women’s History Month, we are recognizing females in life science leadership roles who are making a difference in the industry and on women’s health. Rachel Wellner, MD, MPH, FACS, is the co-founder and CEO of Caelum Diagnostic Solutions, Inc. (CDS), an early-stage oncology company based in Los Angeles that is enabling real-time tissue biopsy diagnostic results for cancer, eliminating the time in between a patient has a biopsy done and learning if they have cancer or not. Prior to starting the company, Dr. Wellner had a long career as a breast surgeon. She talked with us about the moment she realized that a new imaging technology could become a promising diagnostic tool for not only detecting cancer but studying its rate of growth and other factors that help clinicians plan treatment options.
Caelum Diagnostics imagines a world in which patients don’t have to wait for a cancer diagnosis. Can you explain how the company is changing the procedure for tissue analysis and diagnosis?
Currently, people must wait anywhere between two to five days to receive their diagnosis, and that’s for those in an emergency state. For a routine colonoscopy, they’re usually waiting 10 days or more. Either way, it’s a stressful time for the patient who’s waiting to find out if he or she has cancer. And in fact, in the cases where cancer is a high probability, those patients are under severe emotional trauma. It then causes an emotional domino effect starting with the anxiety and fear after finding out which can cause issues with relationships, and family members are under so much stress and worry for the patient that it affects everybody.
In the world we live in, there is no system to make that diagnosis rapid. To solve for this, Caelum Diagnostic Solutions is creating a device that provides an immediate 10-minute biopsy. At the heart of our technology is fluorescence lifetime imaging microscopy (FLIM), which allows for the rapid visualization of images to distinguish between cancer and non-cancer with 94 to 99 percent accuracy. While the patient is undergoing his or her biopsy procedure, there is a technician putting the specimen underneath the machine, and receiving an answer while that patient is still undergoing biopsies right by the bedside or in an adjacent room. Often times, multiple biopsy samples are each being tested in rapid succession such that by the time the patient is bandaged up, we have an answer.
With the significantly accelerated time patients will receive results, how can this improve their outcome?
While there are currently a few ways to analyze tissue quickly, they’re massively inferior to what we’re trying to do which could greatly improve a patient’s outcome. We’re able to give patients 99 percent accuracy, not 50 percent accuracy, or a likelihood versus and unlikelihood that there’s cancer. We’re looking to make this almost, if not as good as standard histopathology. That will make all the difference in the world and will allow patients to start coming up with treatment plans much earlier. We can determine more quickly whether they’re a surgical candidate or a medical oncology candidate. Some patients are not candidates for surgery and need to have chemotherapy first to shrink a tumor or for other reasons. This is the information that we’re hoping provide with that early biopsy information.
With the rate of cancer growing by approximately 2.5 percent each year and more biopsies being performed, how is that impacting patients, clinicians at hospitals?
Since the 1970s, the rate of cancer has been growing rapidly. This is partially because it truly is growing and partially because we’re getting much better with screening tests and diagnosing it more frequently. In any case, these numbers are becoming overwhelming. Right now, there are about 20 million cases in the world per year, which is an underestimate as many countries don’t keep accurate registries of the cancer incidents. By 2040, it’s expected to be 30 million cases out of a population of about nine to ten billion people on the planet, which is astounding. As the population grows, the need for rapid biopsies will go up.
The problem is, there is a fixed number of hospitals and medical centers that must accommodate the growing number of patients. When a hospital is putting together its operating room schedule and scheduling three patients versus seven patients per day to operate on, that accelerated information would help them with their flow a great deal. It would help them to cluster those patients and treat as many as possible, which has a twofold effect. One is you’re treating a lot more patients more quickly and from a revenue perspective, that hospital is increasing their revenue by being able to operate on more patients in that same period of time. This is true of medical oncology as well. The more patients they can get in to start treating with the chemotherapy faster, the better.
You had a successful proof of concept in September of last year at UCLA. What other milestones must be met for clinicians to utilize the diagnostics technology?
Yes, we had a positive proof concept that showed that we can generate a 10-minute biopsy. This has been done in research centers, but no one has translated that into the clinical setting so we’re working fast and furious to get this out as soon as possible. We have submitted our main patent and we’re waiting to hear back about approval.
Once we create a prototype, we will start learning on the machine and I believe we can go straight to clinical testing. We’ve already tested on a murine model that we induced with human cancer within the mouse to prove our concept. We determined there was no damage to tissue whatsoever, no degradation of the image with time, and no interruption of clinical flow. Putting it under this different model FLIM is what we basically did. The one that we use will require even less wattage, less power, and less possibility for damage which is why I believe that we’ll be permitted to take this right into the clinical space. We will start with breast cancer so as patients are having their breast cancer operations, we would take the tissue and evaluate it with what we’re calling our RPIDxTM device, which stands for Real-time Pathology Intra-procedural Diagnosis.
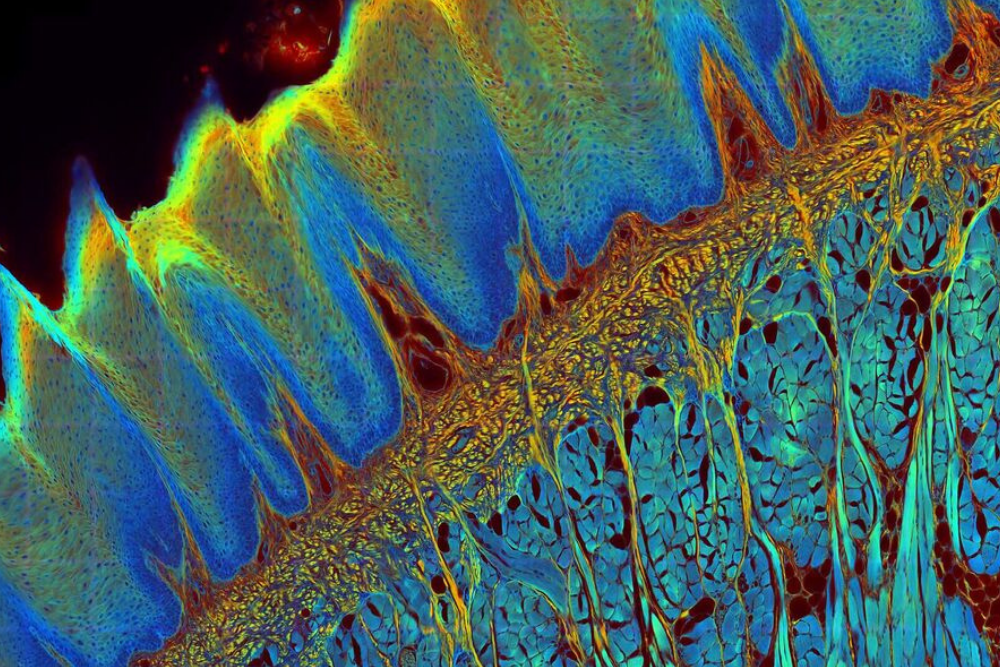
Fluorescence lifetime imaging microscopy (FLIM) is an imaging technique based on the differences in the exponential decay rate of the photon emission of a fluorophore from a sample.
Are there other ways this technology can help clinicians better understand cancer?
Yes, with the genetic information tumors contain, we’re able to tell how differentiated the tumor is, meaning how close it is to the native tissue, how far away it is from the native tissue, or how fast the cancer’s growing. This information helps determine whether a patient needs chemotherapy or not.
Understanding the metabolism of cancer and knowing if a patient has a really slow growing breast tumor, for instance, a clinician could determine that the patient doesn’t need chemotherapy, they just need hormonal therapy. Seeing that there’s a fast-growing cancer, you would need chemotherapy right away, and you could also know which types of chemotherapies would work. An ideal state would be that the minute that biopsy comes out, in addition to knowing if there’s cancer, you would know if the tumor is well differentiated or poorly differentiated, sensitive to certain types of chemotherapy, or if there was a genetic defect like the BRCA gene. Being able to give that information to the patient and then planning their entire treatment out, while looking at the whole scope of what they’re about to go through, is extremely valuable and could help keep healthcare costs down.
After speaking with clinicians and patients about having this capability what has been the response so far?
We’re currently surveying clinicians to determine that our target customers need this device. I actually have one advisor who is a breast cancer survivor and a patient advocate/speaker/author who has become our ambassador going to the patient advocacy groups and talking to key opinion leaders to obtain statements from them regarding the absolute need for something like this. Just the other day I had an Instagram post talking about what we’re doing, and I had 600 people just come right out and say, ‘We need this, we needed this yesterday.’ It’s a good cross section of people that agree about the importance of having this available.
Let’s shift over to your career path. You’ve spent a majority of your career as a breast surgeon, what motivated you to transition over to founding Caelum Diagnostics?
I had a robust career as a breast surgeon, and honestly, I loved it. But when I was sitting in a research conference and saw the use of the FLIM technology for the first time in my life, I was just like enthralled with what was being done. The ability to visualize cancer in that way was spectacular. From there, I got the idea for intraoperative margins. So, during a breast cancer operation, if they’re not doing a full mastectomy and doing a lumpectomy, taking a piece of the breast, we don’t know if we’ve removed all of the cancer until a week later. Same idea, right? The pathologist must look at every side of the breast cancer, all the margins, and make sure that there’s nothing still in the patient’s body. So that was my first concept of what to do with this FLIM technology, and to create a system around it with its laser camera. Interestingly, in the last year or so, FLIM has increased their indication to be able to see margins. When I started, it couldn’t see that much tissue. I then pivoted the company into the diagnostic space, recognizing that that’s just as big of, if not a far greater need.
During my medical school, I got my master’s in public health because I had this sincere desire to help the world and before that I started medical missions in Nicaragua, the Dominican Republic, Guatemala, and India. I always wanted to help the world’s underserved, and anybody who’s in crisis. As soon as I saw that technology, I knew that I had to dedicate a portion of my life to trying to figure this out. This is a legacy that will stick around forever and will advance the field as a whole and not treat just one patient at a time.

With March being Women’s History Month, what does this mean to you, and what advice do you have for women working in healthcare and the life sciences?
First of all, thank you for choosing to feature me and Caelum Diagnostics during Women’s History Month—that is a huge honor. I grew up in the 70’s, shortly after the women’s movements which followed a very militant era that wouldn’t allow someone like me to even think about becoming a surgeon or a doctor. There were these pioneering women that would do it. It was rare to see one in a training program. I was lucky enough to have five women in my class of six. I also had a mom who never made me feel like opportunities were limited for me because I was a woman. We need to teach our little girls and make women aware that every opportunity is open to them no matter what.
I know that the progress we’ve made couldn’t have happened without women like Susan B. Anthony, Gloria Steinem, or Shirley Chisholm. These women were willing to shoot for the stars—whether they made it or not—they did it. And their legacy allows us to shoot for the stars.
What is the biggest challenge Caelum is facing right now?
We’re moving ahead at a steady pace, but the bottleneck is receiving our seed fund. We’re looking for $1.5 million to help build a prototype with the team we have in place and to outsource to both hardware and software engineers. The seed funding will also help when we involve instrumentation companies and to cover all the costs involved when we begin early trials. The trial fees include working with the institutional review board, fees for conducting the trials, and the thousands of whole market surveys we would need to hand out. After our seed round we’ll be looking to mass manufacture and outsource the manufacturing.
What’s on the horizon for Caelum Diagnostics this year, and what are your plans for the future of the company?
This year, we need a prototype, clinical trials, and we are planning to do an FDA pre-submission. Once we get our prototype and start testing our algorithms, I’d like to submit for three to five more patents. We’re also aiming to survey those who are both our target customers and those who are not our target customers. For instance, medical oncologists are not our target customers; they are influencers. They’re not buying this machine because they don’t do biopsies, but their input on why this would be of value to them is important. With some of the additions to the team that we’ve had in recent weeks, we’re creating an uber team, but we also have more key hires planned for the future.