DC Fly-In Library
Emerging Technologies Fly-In
April 29 – May 1, 2024
This year’s participant group was comprised of innovative medical device, artificial intelligence (AI) and diagnostic companies that advocated for policies impacting emerging technologies. Participants included CEOs and senior-level executives from small- to medium-sized companies throughout the world. We had representation from members in San Diego, Los Angeles and the Bay Area, as well as abroad in London and Sydney.
Participants first met with Saif Khan, Senior Advisor to the Secretary for Critical and Emerging Technologies, Department of Commerce, to discuss the White House’s Executive Order on AI and the use of AI by our industry. Next, the group met with Tamara Syrek-Jensen, Director of CMS’ Coverage and Analysis Group (CAG) and her team to learn about the different ways to receive coverage for devices and diagnostics and how to engage with CMS. Last but not least, participants met with Dr. Jeff Shuren, Director of FDA’s Center for Devices and Radiological Health (CDRH) and his team to discuss many programs and initiatives important to our industry, including the Total Product Life Cycle Pilot (TAP), Home as a Health Care Hub, repurposing and health equity.
The final day brought participants to the halls of Congress, where they were met by staff of the Senate Health, Education, Labor and Pensions (HELP) Committee to discuss regulatory policy and the work of the committee to foster innovation. On the House of Representatives side, we were grateful to have intimate conversations with Congressman Kevin Mullin (Bay Area) about the growth of our industry, Congressman Ted Lieu (Los Angeles) about his work on AI policy and the senior staff of Congresswoman Sara Jacobs (San Diego) about home health diagnostics.
Energy and Agriculture Fly-In
May 8 – May 10, 2023
We kicked off our 2023 fly-in with a welcome dinner which allowed participants to network, share their priorities and goals for the event, and prepare for upcoming meetings. This year’s participants represented small and large companies from throughout California working in cellular agriculture, plant-based foods, plant-based material and innovative crops and traits.
On day two, participants had the unique opportunity to discuss the President’s Biotechnology and Biomanufacturing Initiative, access to capital, manufacturing & scaling-up, and labeling with officials at the White House’s Office of Science and Technology Policy (OSTP), Environmental Protection Agency (EPA), Food and Drug Administration (FDA), and United States Department of Agriculture (USDA).
On day three, participants advocated for changes to USDA’s loan guarantee program and biopreferred program in the Farm Bill, which is being reauthorized this year for the next five years. They met with the House Agriculture Committee, Rep. Scott Peters, as well as the offices of Reps. Salud Carbajal, John Duarte, and Doug LaMalfa.

Rare Disease Fly-In
March 9-11, 2020
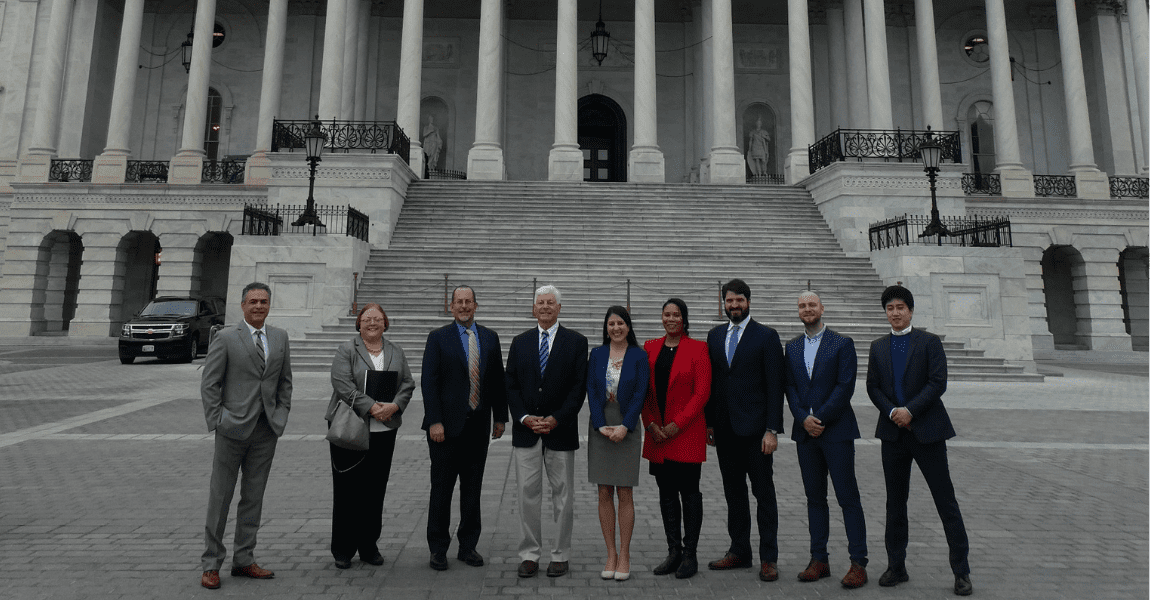
This year’s participants advocated for advancing research and development (R&D) for rare disease products, just a few days after Rare Disease Day. Fly-In participants advocated for protecting the Orphan Drug Act of 1983 (ODA), which provides incentives to innovators for developing products with orphan designations. According to the National Organization for Rare Disorders (NORD), only 34 therapies for rare diseases existed before the law’s passage; as of 2019, there were more than 770 therapies available to treat rare diseases. Yet, in 2017, Congress reduced the Orphan Drug Tax Credit by 50%. Participants also learned about programs at the Food and Drug Administration (FDA) and the National Institutes of Health (NIH) that provide resources, assistance and funding for orphan product R&D.
On the second day, participants had the opportunity to meet with officials at FDA, including at the Office of Orphan Products and Development (OOPD) and the Rare Diseases Program in the Office of New Drugs (OND) at the Center for Drug Evaluation and Research (CDER), as well as at NIH, including at the Office of Rare Disease Research (ORDR) at the National Center for Advancing Translational Sciences (NCATS) and the Small Business Education and Entrepreneurial Development (SEED) Office. They also met with Congressmen Mike Levin and Scott Peters of San Diego and congressional staff from the offices of Sen. Kamala Harris and Rep. Jimmy Gomez.
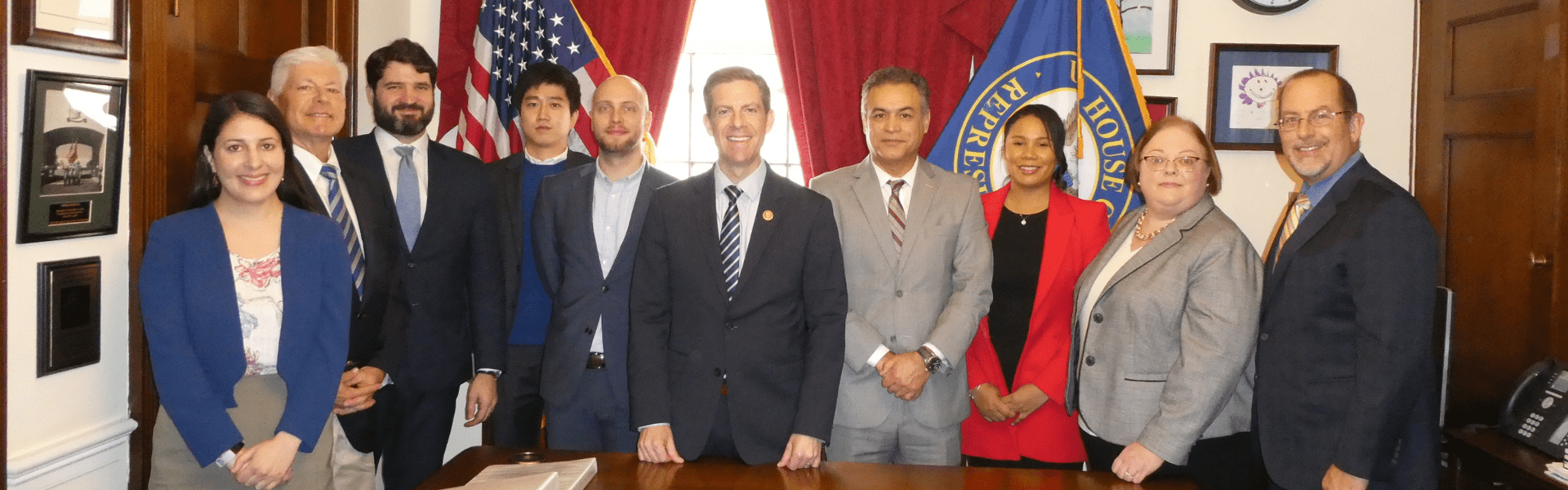
Connected Health Fly-In
March 11-13, 2019
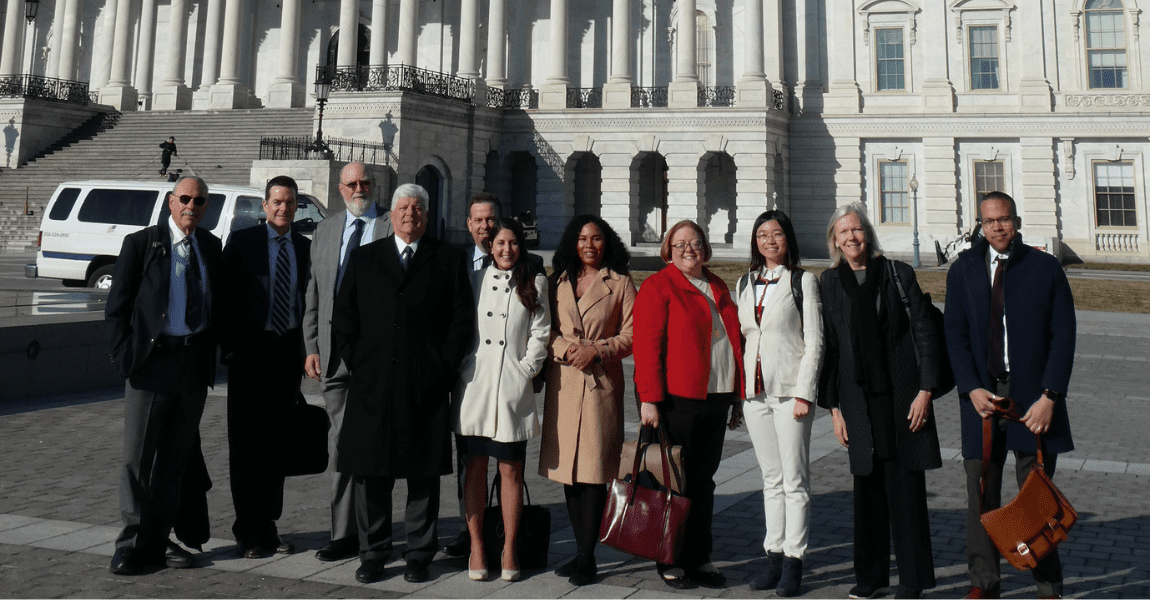
Connected health harnesses the power of technology to improve health care delivery and outcomes, and lower healthcare costs. This year’s Fly-In participants worked on a broad range of issues, from pain management and medication adherence to DNA databases and big data analysis. Together, they advocated for regulatory, reimbursement and data privacy and security policies that incentivize the development and use of connected health technologies. Increasing funding for the National Institutes of Health (NIH) and permanently repealing the medical device tax were also among the top priorities.
Participants had the opportunity to meet with officials at the Food and Drug Administration (FDA), including the director of the Center for Devices and Radiologic Health (CDRH); the NIH, including the Office of the Director and the National Institute of General Medical Sciences (NIGMS); and the executive director of policy at the Office of the National Coordinator for Health Information Technology (ONC). In Congress, they met with the Senate Finance Committee, House Energy & Commerce Committee, Rep. Scott Peters (D-CA-52) and Rep. Mike Levin’s staff (D-CA-49).
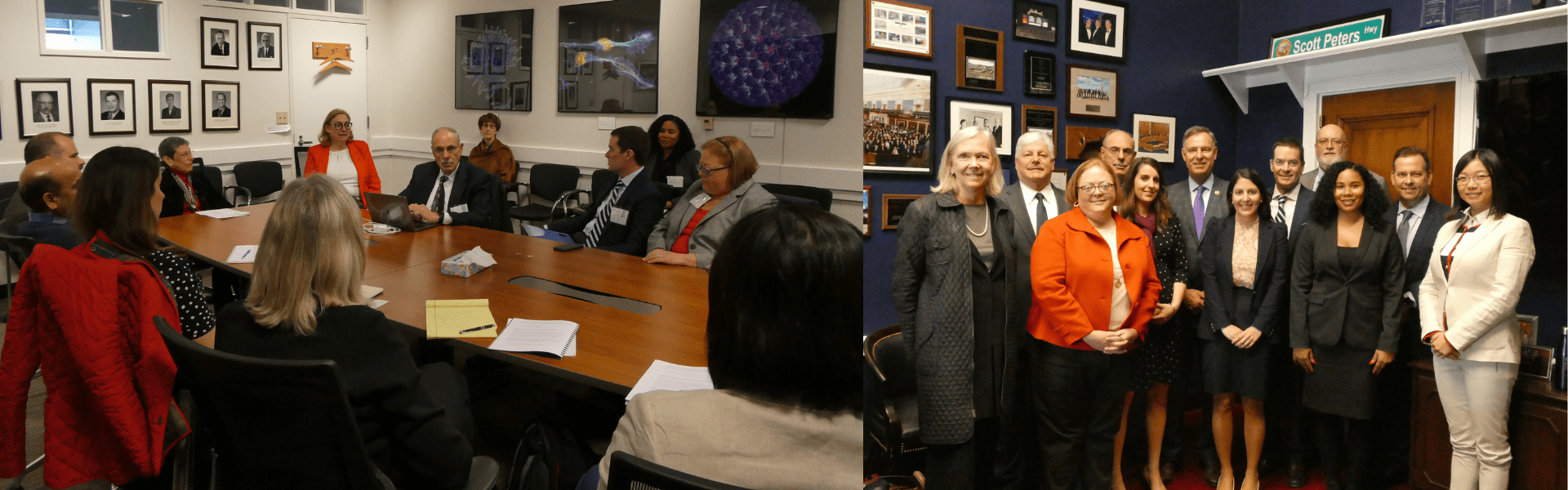
Regenerative Medicine Fly-In
March 11-13, 2018
Regenerative medicine is an emerging field of research that is pioneering ways to regrow, repair or replace human cells, tissues and organs that have lost function due to age, disease, damage or congenital effects. Researchers can either stimulate previously irreparable tissues and organs to heal themselves or grow them in the laboratory and safely implant them into the body when it cannot heal itself. Biocom California’s Regenerative Medicine Fly-In brought 10 participants from seven companies, including researchers, regulatory experts and business executives to Washington to meet with officials at the National Institutes of Health (NIH) and the Food and Drug Administration (FDA), as well as U.S. Representatives Susan Davis, Scott Peters, Lou Correa and Judy Chu. Participants had the unique opportunity to discuss new advances in regenerative medicine and the successes and challenges they face.
This fly-in topped almost a year of work within the organization to advance California’s regenerative medicine space. Biocom California created and led a coalition of research institutes and companies to analyze the current regenerative landscape, identify challenges and provide recommendations to the FDA. Last month, Biocom California submitted several comments to the drug agency providing insight into what needs to be done to support and advance regenerative medicine research in California and across the country.

Oncology Fly-In
April 3-5, 2017

This year we took a group of small companies working in oncology to Washington, D.C., as part of our 2017 Oncology Advocacy Fly-In. Biocom California Fly-In participants had the opportunity to meet with officials at the Food and Drug Administration (FDA), including the director of the Center for Devices and Radiologic Health (CDRH), the National Cancer Institute’s (NCI) SBIR/STTR program, the Centers for Medicare and Medicaid Services (CMS) and key California legislative offices, to discuss new advances in cancer research and the challenges they face to develop these life-saving therapies and products.
Seven participants from six companies focused on cancer research participated in the event, including early-, mid- and commercial-stage companies developing therapeutics and diagnostics. Their treatments and technologies offer the potential promise of treating cancer using genetics to identify the most effective therapies and could lead to higher survivability at lower cost to the healthcare system.